A Tiny Evolutionary Change That Weakens Our Cancer Defenses
This human-specific genetic change weakens FasL, reducing immune attack on solid tumors. Blocking this effect may boost cancer immunotherapy, offering new hope for hard-to-treat cancers.

AI Research Assistant
Analyze this article instantly
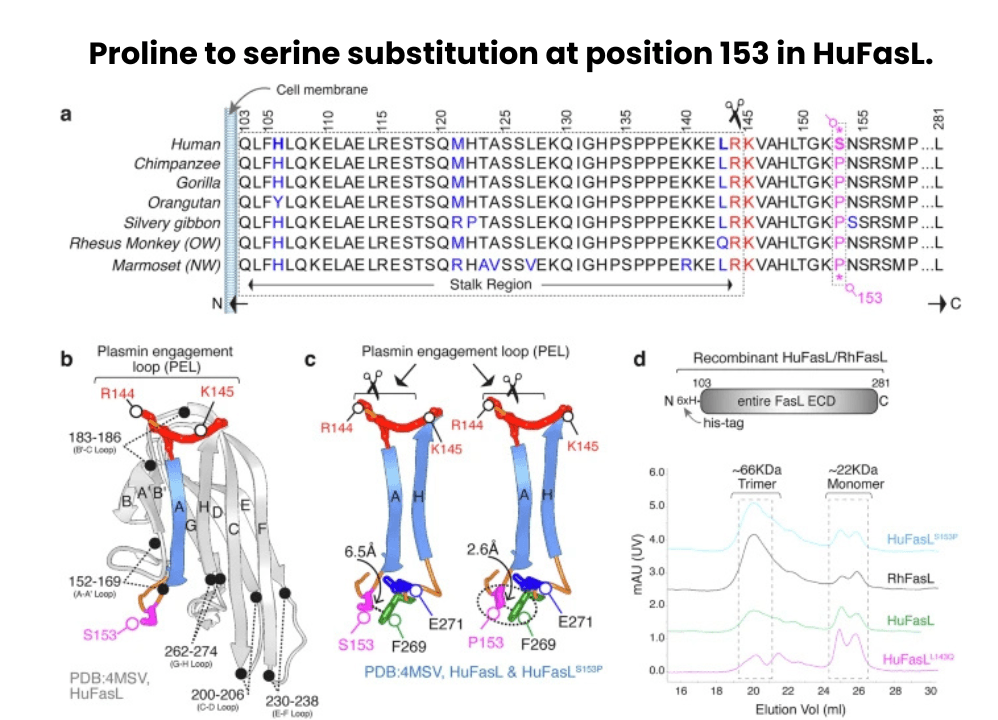
Another new discovery that bridges evolutionary biology with critical cancer therapy, a team of international scientists led by Dr. Jogender Tushir-Singh at the University of California, Davis has revealed how a small, human-specific change in a key immune protein could be one reason why immunotherapy doesn’t work as well in solid tumors. The findings, published in Nature Communications, explore how a single genetic substitution in humans - a switch from proline (P) to serine (S) at position 153 in the Fas Ligand (FasL) protein - might be undermining the effectiveness of T-cell-based cancer treatments.
FasL is a powerful protein found on the surface of T-lymphocytes and CAR-T cells, responsible for triggering the death of targeted cancer cells by engaging the Fas receptor (CD95). This process, known as extrinsic apoptosis, is one of the immune system’s most effective strategies for eliminating dangerous or abnormal cells. But in humans, the presence of serine at position 153 makes FasL unusually vulnerable to cleavage by plasmin- a protease enzyme that is often elevated in the microenvironment of solid tumors, such as ovarian and colorectal cancers.
Source: https://www.nature.com/articles/s41467-025-60990-0/figures/1
Once cleaved by plasmin, FasL loses its ability to form the strong, trimeric structures needed to activate death signals in cancer cells. As a result, even when T-cells or CAR-T cells infiltrate tumors, they may fail to kill them efficiently if FasL has already been inactivated by tumor-derived plasmin. Interestingly, this vulnerability does not exist in chimpanzees and other non-human primates, where FasL retains the ancestral proline (P153), which structurally protects the protein from plasmin-mediated degradation.
Turning a Molecular Weakness into a Therapeutic Opportunity
To confirm this, the researchers recreated human and primate versions of FasL in the lab and exposed them to plasmin. The human version was selectively cleaved, while the chimpanzee version remained intact and functional. Further, when the human Ser153 was replaced with Pro153 (mimicking the primate version), the FasL protein became resistant to plasmin, restoring its cell-killing ability. These elegant molecular experiments offer a clear mechanistic link between a specific evolutionary mutation and impaired immune response in cancer.
But the story doesn’t end there. The researchers took this discovery a step further by designing targeted antibodies (Nok2h and 9F5) that bind to FasL and shield it from plasmin attack. When used in combination with CAR-T cells or immune checkpoint inhibitors (like anti-PD-L1 therapy), these antibodies rescued FasL activity and significantly enhanced tumor killing in mouse models. In tumors rich in plasmin activity-which normally block FasL-mediated killing - the combination therapy led to better caspase activation, immune cell infiltration, and tumor regression.
Perhaps most exciting is the broader implication: this is the first time a human-specific evolutionary variation has been directly tied to reduced immunotherapy effectiveness. The study helps explain why T-cell therapies often succeed in blood cancers, where plasmin levels are low, but struggle in solid tumors, where plasmin is abundant and actively neutralizes immune signalling.
Moreover, the study demonstrates that FasL’s function is not just dictated by the immune cells, but also by how tumor cells can manipulate the environment - in this case, by secreting enzymes that disable immune weapons. It also emphasizes the need to tailor cancer immunotherapies not only to tumor type but also to the molecular ‘terrain’ of the tumor microenvironment.
Also Read: New research offers new hope for longer healthier lives
In essence, this discovery opens up a new strategy for strengthening immunotherapies by co-targeting tumor-derived proteases like plasmin or by engineering protease-resistant immune molecules. It’s a powerful example of how deep molecular understanding - informed by evolutionary biology - can help overcome barriers in modern cancer treatment, especially in aggressive and treatment-resistant solid tumours.



