AI-enhanced Genome Editing: How CRISPR-Cas9 and Machine Learning are Shaping the Future of Medicine
CRISPR-Cas9, enhanced by AI, transforms from bacterial defense to clinical tool, advancing genetic disease treatment, drug design, and vaccine creation.

AI Research Assistant
Analyze this article instantly
In a lab in Boston, scientists watch a simulation unfold on a computer screen. On one side: a DNA sequence associated with a hereditary disorder. On the other: a digital projection of how an AI-designed guide RNA directs the Cas9 protein to snip the faulty region. It’s a clean match - and a safe one. This moment captures the new frontier in biomedical science: the convergence of CRISPR-Cas9 and artificial intelligence (AI). CRISPR, once a humble bacterial immune system, has evolved into the go-to genome editing tool for researchers around the globe. But it’s AI that is refining its edges, helping scientists avoid risks, predict outcomes, and design smarter therapies. “We’ve known for a while that CRISPR is powerful,” says Dr. Li, a computational biologist at the forefront of gene editing software. “But now, with AI, it’s also becoming precise.”
Also Read: AI in Material Discovery
From Bacteria to Breakthroughs
The CRISPR-Cas9 system was first discovered in bacteria, where it served as a defense against invading viruses. Researchers soon realized they could reprogram this natural defense mechanism into a highly targeted genetic editing tool. The system consists of two key parts: the Cas9 enzyme-the molecular scissors-and a guide RNA (gRNA), which directs the scissors to a specific location in the genome, using a short DNA motif known as PAM to lock in. Once the DNA is cut, the cell repairs it either through non-homologous end joining (NHEJ), which is quick but often messy, or homology-directed repair (HDR), which is more accurate but less efficient. This dual-pathway repair system opened up possibilities in medicine, agriculture, and synthetic biology - but also raised concerns about control and safety. Dr Mengstie et.al emphasized that the CRISPR-Cas9 system effectively repurposes the cell's endogenous repair mechanisms, a process that inherently introduces an element of unpredictability due to the reliance on cellular pathways like NHEJ and HDR.
Precision Needed: The Challenge of Off-Target Effects
Despite its promise, CRISPR is far from perfect. One of its greatest limitations has been off-target effects, where Cas9 mistakenly cuts similar, but unintended, sequences in the genome. These unintended edits can have serious consequences, particularly in therapeutic applications.
That’s where AI comes into play.
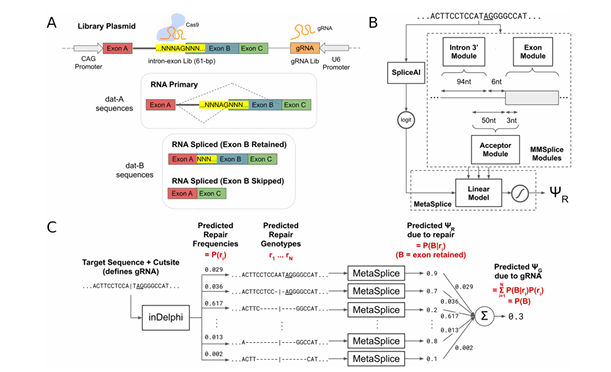
Source: Machine learning based CRISPR gRNA design for therapeutic exon skipping | PLOS Computational Biology
Machine learning models, trained on vast datasets of known CRISPR interactions, can now predict the most likely off-target sites before a single experiment begins. AI tools help design better gRNAs, optimize Cas9 variants, and even suggest alternative genomic targets with fewer risks. “It’s like running a flight simulator for a genome edit,” says Dr. Lin, who develops AI-powered CRISPR platforms. “We can test thousands of scenarios without ever entering the wet lab.”
Also Read: The AI-Powered Shortcut to Next-Generation Protein Engineeri...
Redesigning the Scissors
But AI isn’t just improving where CRISPR cuts - it’s also changing how it cuts.
Dr. Uddin et.al are using deep learning models to evolve new forms of Cas9 that are smaller, more specific, and less likely to trigger an immune response. He says “The original Cas9 protein came from bacteria, so our immune system recognizes it as foreign,”. “We’re now using AI to ‘humanize’ the protein -to reduce those red flags.” Other teams are exploring alternative CRISPR systems - such as Cas12, Cas13, and even base editors-that offer different kinds of genetic modifications. AI is proving essential in comparing, testing, and optimizing these complex tools for various therapeutic purposes.
From Sickle Cell to Personalized Vaccines
In the clinic, CRISPR-Cas9 is already showing promise. Trials are underway to correct mutations responsible for sickle cell anemia, beta-thalassemia, and hereditary blindness. In oncology, CRISPR is being used to edit immune cells so they can better attack tumors. AI helps identify which genes to target for maximum therapeutic effect. In the era of COVID-19, CRISPR was also used to develop faster diagnostic kits and AI-enhanced platforms for vaccine design reported by Dr Akbar et.al. By scanning viral genomes, AI models predicted the most stable and immunogenic targets- guiding the development of mRNA vaccines in record time. Another emerging field is exon skipping, where AI algorithms help reprogram gene splicing to skip over mutated segments. “We're not just editing the genome anymore,” says Dr. Louie, who works on RNA-level interventions. “We’re editing the way genes behave.”
Also Read: AI is changing the future of medicine.
Yet challenges remain. Delivering CRISPR components into the right cells is a significant hurdle. Viral vectors can trigger immune responses; nanoparticles may not reach their targets efficiently. AI is being used to optimize delivery vehicles, tailoring them for tissue-specific targeting and improved safety. The immune system's response to CRISPR - particularly the bacterial origins of Cas9 - is another concern. Kim et.al are using AI to design engineered or de-immunized variants of Cas9 that retain cutting efficiency but are less likely to provoke inflammation or rejection.
AI as the Co-Pilot of Genome Editing
As CRISPR tools become more complex, Dr Abbasi and team explored that AI will evolve into a full co-pilot for genome engineering - able to model cellular behavior, predict editing outcomes, and even automate experimental workflows. They say that “The goal is to move from trial-and-error to a predictive, simulation-driven model of gene therapy,”. Dr Muhammad & Dr Andreas is developing AI models that can predict not only where Cas9 will cut, but also how a cell will react, repair, and express genes after the edit. Such predictive capacity could revolutionize personalized medicine - tailoring treatments to each individual’s unique genome, disease profile, and risk factors.
But unlocking this potential will require more than just better algorithms. Robust regulatory frameworks, equitable access, and sustained public investment are vital. Samuel H. Sternberg et.al worry that funding cuts- like those seen in the U.S. BRAIN Initiative- could slow progress just as AI-CRISPR integration reaches a tipping point. “There’s a real danger that we build the tools but fail to deploy them,” warns Dr. Sternberg, who helped uncover the structural biology of Cas9. Ethical concerns also loom - from germline editing to data privacy. The global research community is still debating where to draw the line, particularly as AI could accelerate both therapeutic and non-therapeutic uses of gene editing.
A Future Within Reach
Despite the complexities, the future looks promising. AI is transforming CRISPR from a powerful genetic scalpel into a smart, context-aware system capable of tailoring interventions at the individual level. “We are entering the era of ‘predictive editing’,” says Dr. Xue, “where every cut is calculated and personalized.” As clinical trials expand and new partnerships between biotech and AI companies flourish, the vision of safe, precise, and affordable gene editing is becoming real. “This isn’t science fiction anymore,” adds Dr. Sanchez-Rivera, a cancer researcher. “It’s the start of a new chapter in medicine - one where your DNA might be your most important prescription.”



